Starpax is a dual Biopharma / Medtech Company that has developed a revolutionary platform for treating cancer.
- FOR SOLID TUMORS REPRESENTING ≈ 90% OF CANCERS
- INTENDED TO PRESERVE THE TISSUES AND FUNCTIONS OF ORGANS
- CONCEIVED TO AVOID SIDE EFFECTS OF CHEMOTHERAPY, RADIATION THERAPY, IMMUNOTHERAPY AND SURGERIES
About
The innovative Starpax’ technology combines four different science
disciplines simultaneously to treat cancer:
ELECTROMAGNETISM
ENGINEERING
MICROBIOLOGY
ARTIFICIAL
INTELLIGENCE
INTELLIGENCE
BIOCHEMISTRy
The development
Since 2017, more than 450 professionals (MD, oncologists, pharmacokinetic & pharmacodynamic specialists, chemists, biochemists, microbiologists, artificial intelligence experts, electromagnetism and medical device engineers, regulatory experts, biostatisticians, biopharma manufacturing experts, quality professionals) have collaborated to transform a proven working concept of cancer treatment platform into a revolutionary multidisciplinary technology to address solid cancer tumors in humans (representing 90% of cancers) along with multiple other non-cancer diseases.
manufacturing & production
In 2023, Starpax has completed the construction of its new GMP manufacturing plant in Canada for the production of its therapeutic Starpax Magnetodrones™

THE STARPAX PLATFORM
The platform consists of Intertwined components:
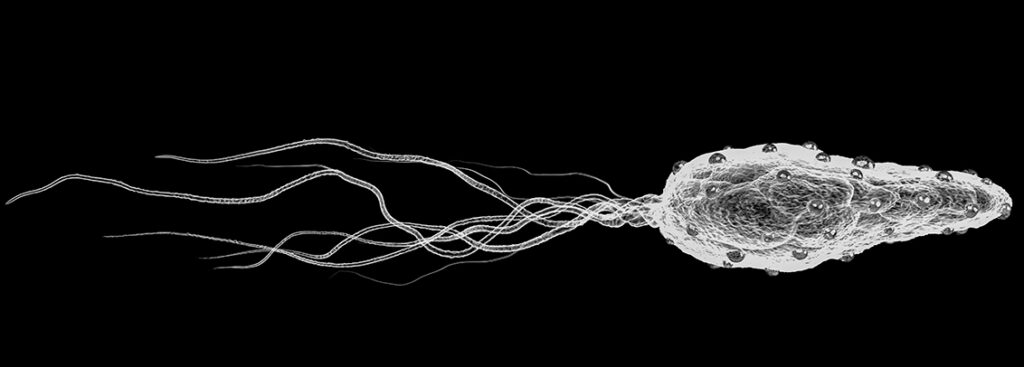
- 1The self propelled Starpax Magnetodrones™
Transporting the therapeutic agent in the tumor without circulating in the blood system,
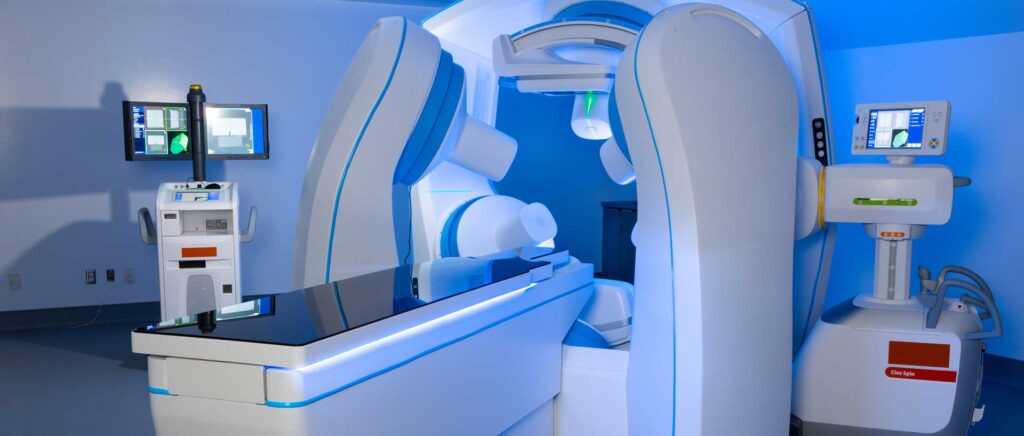
2 The Starpax PolarTrak™
A proprietary medical device that keeps the Starpax Magnetodrones™ captive inside the tumor so they they don’t escape to the rest of the patient’s body, and forces them to distribute throughout the tumor volume, including hypoxic zones where the stem cells are located.

- 1.The self propelled Starpax Magnetodrones™
distributing the therapeutic agents in the tumor without circulating in the blood system.

2. The Starpax PolarTrak®
A proprietary medical device conceived to keep Magnetodrones™ captive inside the tumor. This aiming to prevent them from escaping to the rest of the patient’s body and forces them to distribute throughout the tumor volume; including hypoxic zones where the stem cells are located.
clinical trials
In 2022, the company installed its first PolarTrak® in a hospital and expects to begin its clinical trials in 2024 for Unmet Medical Needs indications in six organs:
- Pancreas
- Prostate
- Head & Neck
- Breast
- Vulva
- Colorectum
New cancers will be added with different already FDA approved drugs as preclinical studies are completed with these new cancers and drugs.
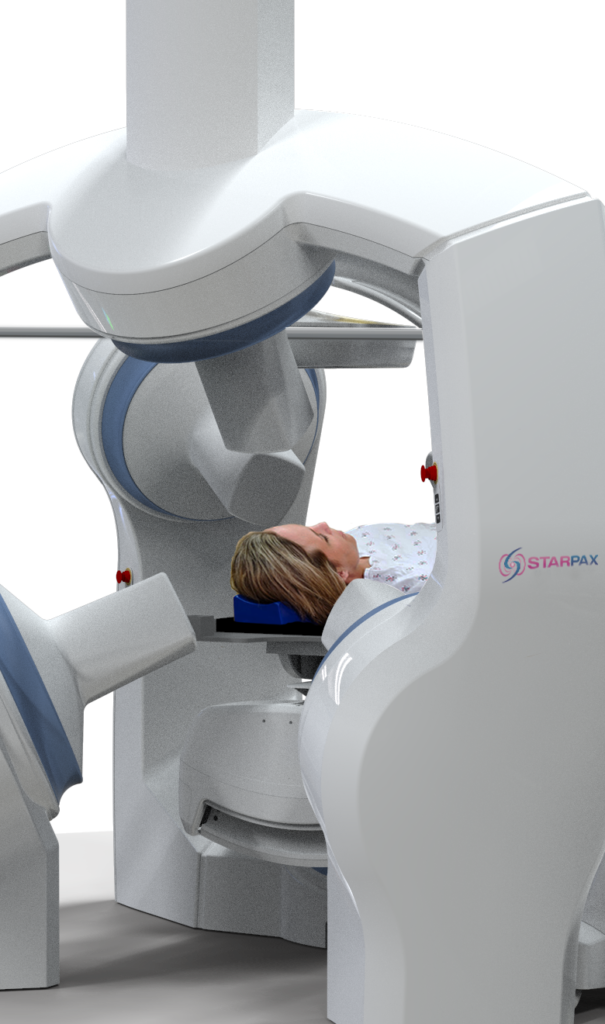
The Starpax Technology is not yet approved for commercial use. Clinical trials are planned to begin in 2024

